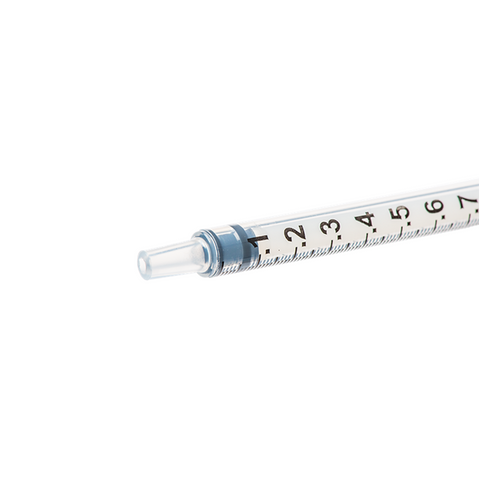
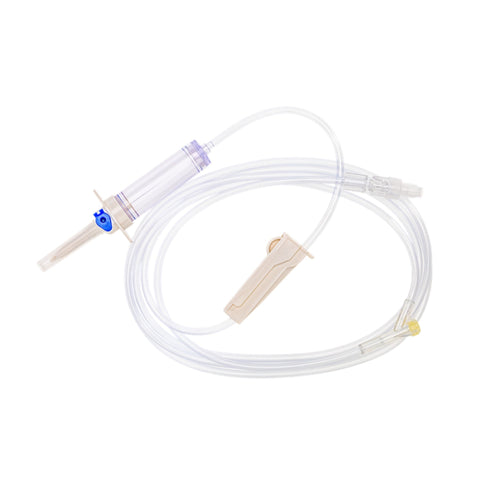
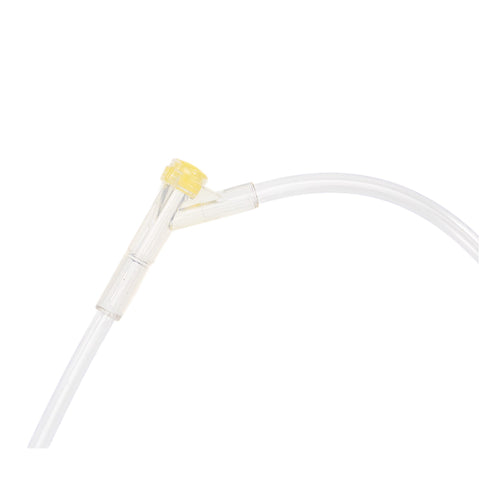
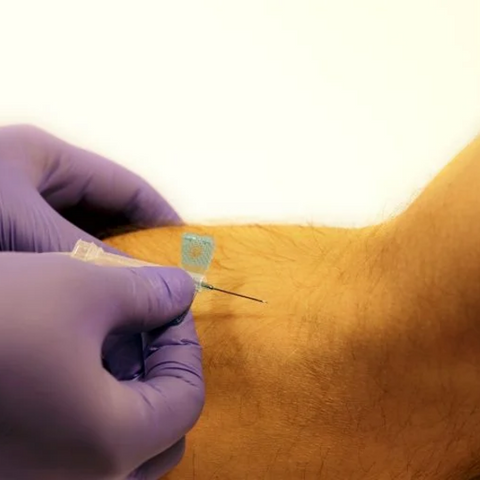
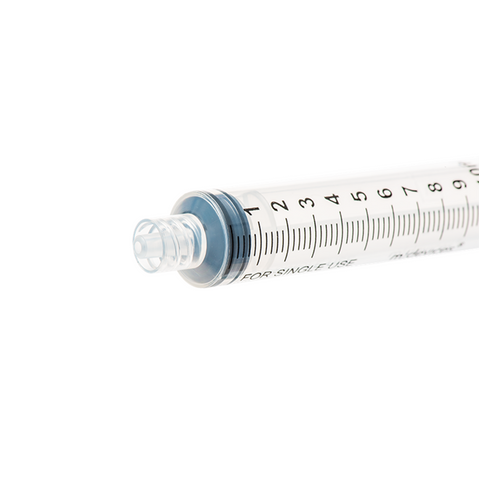
The m|devices NeutralSite™ valve is designed for neutral pressure displacement to prevent blood reflux and reduce the risk of catheter occlusion and catheter-related bloodstream infections (CRBSI). It's swabable flat surface with a pre-slit septum allows for easy disinfection and minimises microbial ingress. The valve features a straight internal fluid pathway to reduce priming volume and minimise dead space concerns. It is recommended for use for up to 7 days based on independent laboratory testing, following industry guidelines for safety and performance.
Features & Specifications:
- Flat swabable pre-slit septum: Easy to disinfect and helps maintain cleanliness.
- Straight internal fluid pathway: Reduces priming volume and addresses dead space concerns.
- Low priming volume: Minimises the amount of fluid needed to activate the valve (0.08mL).
- Neutral pressure displacement: Very minimal reflux volume (0.0059mL), helping to prevent backflow and reduce the risk of occlusion.
- Transparent housing: Provides clear visibility when flushing.
- Gap-less space between septum and housing: Mechanically provides an effective microbial barrier against bacterial contamination.
- Compatible with luer lock and luer slip connectors: Ensures compatibility with common medical devices.
- Maximum flow rate at 350psi: 10mL/second.
- Number of accesses: Over 200 uses.
- PSI rating: 350psi.
- Lipid resistant: Compatible with lipid-based solutions.
- ARTG number: 355439.
Complies with:
- EN ISO 8536-4:2013: Infusion equipment for medical use – Part 4: Infusion sets for single use, gravity feed.
- EN ISO 10993-1:2009: Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process.
- EN ISO 10993-10:2013: Biological evaluation of medical devices – Part 10: Tests for irritation and skin sensitisation.
- DEHP-free: Not detected (result less than the method detection limit EN 14372).
- Not made with natural rubber latex: Safe for latex-sensitive patients.
- Recommended use of up to 7 days: Based on independent laboratory testing.
- Materials: PP, ABS, and silicone.
- MRI compatible: Yes.
Resources:
NeutralSite - Video
Returns Policy
1. Prior Approval Required: All returns must be approved by us prior to sending any goods back. Please contact us to obtain a return authorisation before returning any items.
2. Claims for Issues: Claims for short delivery, incorrect supply, or damaged goods during transport must be made within 7 days of the delivery date. Please inspect your goods upon receipt and notify us immediately if there are any issues.
3. Return Conditions: We generally do not accept returns for any reason other than a faulty product. This includes returns due to change of mind or incorrect orders However, at our discretion and depending on the product, we may accept non-faulty returns. These will incur a 10% restocking fee based on the product's purchase price. Refunds will be processed for the remainder of the order minus the original shipping cost and restocking fee, after the item has been inspected and approved. The buyer is responsible for returning the item in the same condition it was received. We do not accept returns for items that are damaged, opened, or not in original condition.
4. Lost Returns: If a returned item is lost in transit from the buyer to Solmed, we will not be held responsible. Please ensure that you use a trackable and insured shipping method..
5. TGA Duties: For products regulated by the Therapeutic Goods Administration (TGA), returns must comply with relevant TGA regulations and guidelines. Any return of TGA-regulated products will be subject to additional conditions as required by law.
6. Return Shipping Costs: Return shipping costs are the responsibility of the buyer unless otherwise agreed upon in the case of faulty products.
7. If you have any questions regarding returns or need assistance, please contact us before proceeding with the return process.
Shipping
1. Responsibility for Delivery: Once your parcel is with the courier service, it is beyond our control. We are not responsible for any loss, damage, or delivery issues that may occur once the parcel is in the possession of the courier.
2. Authority to Leave: All orders are dispatched without the requirement of a signature. The courier may leave your parcel in a location they deem to be a “safe place.” We cannot be held liable for any loss, damage, or theft that may arise from this decision.
3.Signature on Delivery: For added security, you may select the Signature on Delivery option at checkout. This ensures your parcel will not be left unattended. If no one is available to receive it, the parcel will be taken to the nearest post office or courier depot for collection.
4. Tracking Information: Tracking numbers will be provided to you via email. It is the buyer’s responsibility to monitor the progress of their parcel using the tracking information provided.
5. Buyer’s Responsibility: Any discrepancies or issues with the delivery must be followed up with the respective courier service by the buyer.
6. Transit Cover (Optional): You may purchase transit cover to insure the value of your order in case of loss, damage, or theft. The cost is 1% of your order total. Please contact us if you’d like to add this to your order. This is the only option that provides coverage for your order value.