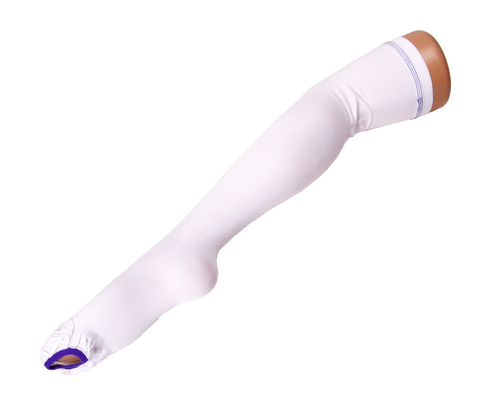
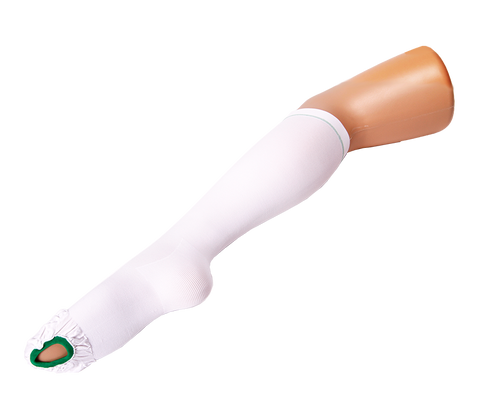
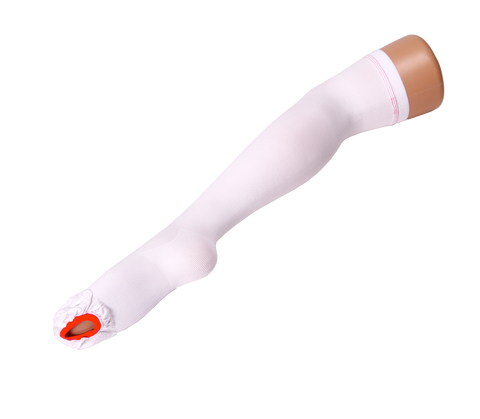
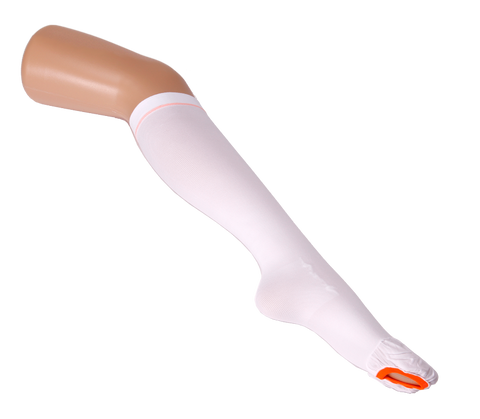
VIENNA™ DVT COMPRESSION STOCKINGS ANTI-EMBOLISM THIGH HIGH MEDIUM ISO BS6612 TESTED
THESE STOCKINGS ARE SIZE MEDIUM FOR CIRCUMFERENCE:
ANKLE SIZE 25-28cm. CALF SIZE 38-45cm THIGH SIZE 56-68cm
Leg Length is 70-80cm
These Compression Stockings help with the prevention of DVT (Deep Vein Thrombosis).
"Please choose size carefully as there is no returns once packaging has been opened
& please consult with your GP to determine suitability regarding your condition"
"SOURCE - SUPPLY - PROTECT"
Another Quality Product from the MEDICAL DEVICES Company SOLMED
Class 3: Firm Compression (40mmHg) at ankle. For serious conditions including severe varicose veins, lymphoedema, post sclerotherapy/vein surgery, healing venous ulcers, deep vein thrombosis or chronic venous insufficiency.
IN HOUSE VALIDATION, CALIBRATION & TESTING OF EACH BATCH TO BS6612
FEATURES:
Hospital Quality
Complies with BS6612 British Standards
Washable in accordance with Standards
Hand Wash Only
Graduated Compression as tested:
Ankle 40mmHg
Calf 32mmHg
Knee 22mmHg
Thigh 14mmHg
Inspection opening on ball of foot allowing feet and toes to be easily accessible for hygiene, peripheral circulation and skin integrity checks
Polyamide and Lycra Elastine Knit with superb wicking properties to move perspiration away from skin keeping you cool & comfortable
Easy Tear Pouch with Instructions & Contraindications
CE Certified
Latex Free
TGA 181646
Distributed by SOLMED ABN 16 132 179 250
Returns Policy
1. Prior Approval Required: All returns must be approved by us prior to sending any goods back. Please contact us to obtain a return authorization before returning any items.
2. Claims for Issues: Claims for short delivery, incorrect supply, or damaged goods during transport must be made within 7 days of the delivery date. Please inspect your goods upon receipt and notify us immediately if there are any issues.
3. Return Conditions: We do not accept returns for any reason other than a faulty product. This includes, but is not limited to, returns due to change of mind or incorrect orders.
4. TGA Duties: For products regulated by the Therapeutic Goods Administration (TGA), returns must comply with relevant TGA regulations and guidelines. Any return of TGA-regulated products will be subject to additional conditions as required by law.
5. Return Shipping Costs: Return shipping costs are the responsibility of the buyer unless otherwise agreed upon in the case of faulty products.
6. If you have any questions regarding returns or need assistance, please contact us before proceeding with the return process.
Shipping
1. Responsibility for Delivery: Once your parcel is with the courier service, it is beyond our control. We are not responsible for any loss, damage, or delivery issues that may occur once the parcel is in the possession of the courier.
2. Safe Place Deliveries: If the courier decides to leave your parcel in a location they deem as a “safe place,” we cannot be held liable for any issues that arise from this decision.
3.Signature Option: For added security, you may request a signature upon delivery for an additional fee of $3.00 per carton. This option ensures that your parcel will not be left unattended and, if no one is available to receive it, will be taken to the nearest post office or depot for pickup.
4. Tracking Information: Tracking numbers will be provided to you via email. It is the buyer’s responsibility to monitor the progress of their parcel using the tracking information provided.
5. Buyer’s Responsibility: Any discrepancies or issues with the delivery must be followed up with the respective courier service by the buyer.