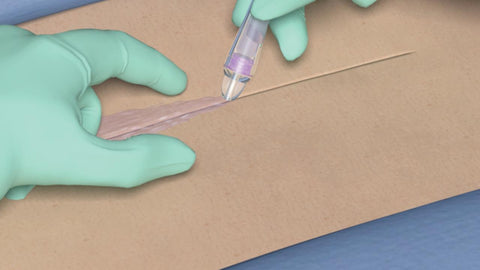
The Prontosan Debridement Pad is specifically designed to provide gentle yet effective soft mechanical debridement for chronic wounds, working in perfect synergy with the Prontosan Wound Irrigation Solution. This product incorporates cutting-edge microfiber technology, delivering unparalleled advantages for wound care and management.
Advantages:
- Superior Cleansing and Debridement: The Debridement Pad's microfiber technology ensures thorough cleansing and debridement of chronic wounds, leaving behind a pristine wound bed and promoting optimal healing conditions.
- Gentle and Non-irritating: The B. Braun Debridement Pad's soft debridement approach ensures no tissue irritation, making the wound healing process more manageable for individuals.
- Eliminates Coatings and Dead Cell Residues: With precision and efficacy, it effectively removes coatings and dead cell residues from the wound site, facilitating better tissue regeneration and minimizing infection risks.
- Versatile Droplet Shape: Featuring a unique droplet shape, enabling the effective debridement of shallow cavities and reaching challenging-to-access areas. This feature ensures comprehensive wound treatment, leaving no areas untreated.
- Secure and Aseptic Soaking: Packaged in blister packs, the Prontosan Debridement Pad guarantees safe and aseptic soaking prior to use. This ensures the integrity of the product and maintains its effectiveness during application.
Range of Application:
Designed for use on chronic wounds, including Pressure Ulcers (PU), Venous Leg Ulcers (VLU), and Diabetic Foot Ulcers (DFU).
Resources:
Returns Policy
1. Prior Approval Required: All returns must be approved by us prior to sending any goods back. Please contact us to obtain a return authorisation before returning any items.
2. Claims for Issues: Claims for short delivery, incorrect supply, or damaged goods during transport must be made within 7 days of the delivery date. Please inspect your goods upon receipt and notify us immediately if there are any issues.
3. Return Conditions: We generally do not accept returns for any reason other than a faulty product. This includes returns due to change of mind or incorrect orders However, at our discretion and depending on the product, we may accept non-faulty returns. These will incur a 10% restocking fee based on the product's purchase price. Refunds will be processed for the remainder of the order minus the original shipping cost and restocking fee, after the item has been inspected and approved. The buyer is responsible for returning the item in the same condition it was received. We do not accept returns for items that are damaged, opened, or not in original condition.
4. Lost Returns: If a returned item is lost in transit from the buyer to Solmed, we will not be held responsible. Please ensure that you use a trackable and insured shipping method..
5. TGA Duties: For products regulated by the Therapeutic Goods Administration (TGA), returns must comply with relevant TGA regulations and guidelines. Any return of TGA-regulated products will be subject to additional conditions as required by law.
6. Return Shipping Costs: Return shipping costs are the responsibility of the buyer unless otherwise agreed upon in the case of faulty products.
7. If you have any questions regarding returns or need assistance, please contact us before proceeding with the return process.
Shipping
1. Responsibility for Delivery: Once your parcel is with the courier service, it is beyond our control. We are not responsible for any loss, damage, or delivery issues that may occur once the parcel is in the possession of the courier.
2. Safe Place Deliveries: If the courier decides to leave your parcel in a location they deem as a “safe place,” we cannot be held liable for any issues that arise from this decision.
3.Signature Option: For added security, you may request a signature upon delivery for an additional fee of $3.00 per carton. This option ensures that your parcel will not be left unattended and, if no one is available to receive it, will be taken to the nearest post office or depot for pickup.
4. Tracking Information: Tracking numbers will be provided to you via email. It is the buyer’s responsibility to monitor the progress of their parcel using the tracking information provided.
5. Buyer’s Responsibility: Any discrepancies or issues with the delivery must be followed up with the respective courier service by the buyer.