Categories
- ADHESIVE DRESSINGS
- ADHESIVE PLASTERS
- ADVANCED WOUND CARE DRESSINGS
- AIDS TO DAILY LIVING
- AIRWAY MANAGEMENT
- ANAESTHETIC MASKS
- ANTI EMBOLISM COMPRESSION DVT STOCKINGS
- ANTISEPTICS
- APRONS
- BANDAGES
- BED PROTECTORS
- BEST SELLERS
- BIOPSY PUNCHES & CURETTES
- BLOOD COLLECTION ACCESSORIES
- BLOOD PRESSURE MONITORS
- BODY WIPES
- BRACES
- BURN PRODUCTS
- CANNULAS
- CAPS
- CATHETERISATION ACCESSORIES
- CLEARANCE - SALE ITEMS
- COMBINE DRESSINGS
- CONTINENCE CARE
- COTTON APPLICATORS
- COVERALLS
- CPR DEVICES
- DEFIBRILLATORS
- DENTAL INSTRUMENTS
- DIAGNOSTIC EQUIPMENT
- DISSECTING KITS
- DRESSING PACKS
- ELECTRODES
- ENDOTRACHEAL TUBES
- ENTERAL FEEDING
- EXTENSION SETS
- EYE & WOUND IRRIGATION
- EYE/EAR WEAR
- FINGER COTS
- FIRST AID EQUIPMENT
- FIRST AID KITS
- FIRST AID SUPPLIES
- FLUIDS
- FORCEPS
- GAUZES
- GELS & CREAMS
- GIVING SETS
- GLOVES
- GOWNS
- GUEDELS
- HAND SANITISERS
- HOLLOWWARE
- HOT & COLD THERAPY
- INSTRUMENT PACKS
- INTRAVENOUS CARE
- IRRIGATION
- IV ACCESSORIES
- LARYNGEAL MASK AIRWAYS
- LARYNGOSCOPES
- LUBRICANTS
- MASKS
- MOBILITY
- NASOPHARYNGEAL
- NEEDLE HOLDERS
- NEEDLES
- NEW ARRIVALS
- NON-ADHERENT PADS
- NURSES EQUIPMENT
- OPHTHALMOSCOPES
- OTHER
- OTOSCOPES
- OXYGEN THERAPY
- PEN LIGHTS
- PROBES
- PROTECTIVE GARMENTS
- Protective Underwear
- PULSE OXIMETERS
- RAZORS
- RECORDING CHART PAPER
- RESPIRATORY / RESUSCITATION
- RESPIRATORY DEVICES
- RESUSCITATORS
- ROLLATORS
- SCALPELS & BLADES
- SCISSORS
- SHARPS CONTAINERS
- SHOE COVERS
- SHOWER
- SKIN & SURFACE PREPS
- SLEEVE PROTECTORS
- SPECULAS
- SPRAYS
- STERILISATION POUCHES
- STETHOSCOPES
- SUCTION TUBING
- SURFACE WIPES
- SURGICAL INSTRUMENTS
- SURGICAL PENS
- SUTURE KITS
- SUTURES
- SWABS
- SYRINGES
- TAPES
- THERMOMETERS
- TOILET
- TOURNIQUETS
- TOWELS
- TUBING CLAMPS
- ULTRASOUND GEL
- URINE BAGS
- URINE CATHETERS
- URINE COLLECTORS
- WALKING STICKS
- WHEELCHAIRS
- WINGED INFUSION SETS
- WOUND CARE
- WOUND PADS
THE PARAMEDIC'S FIRST CHOICE AND SAME GLOVE AS SUPPLIED TO MOST HOSPITAL ACROSS AUSTRALIA
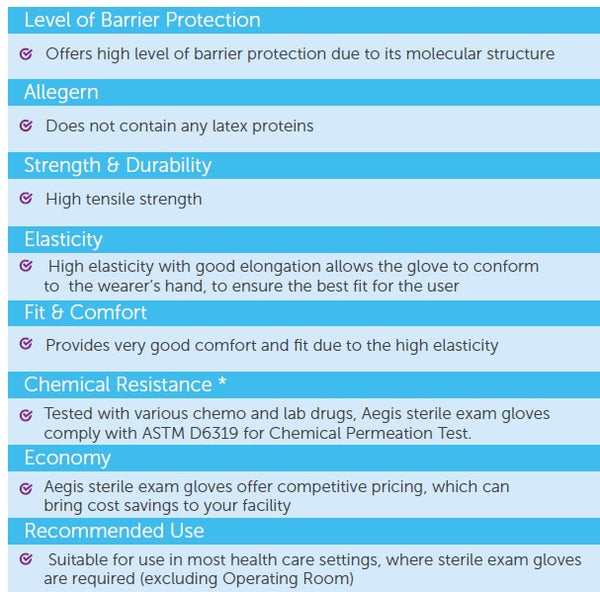
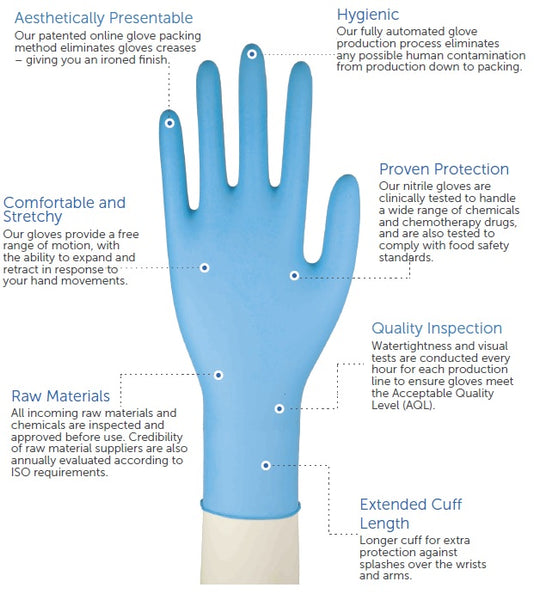
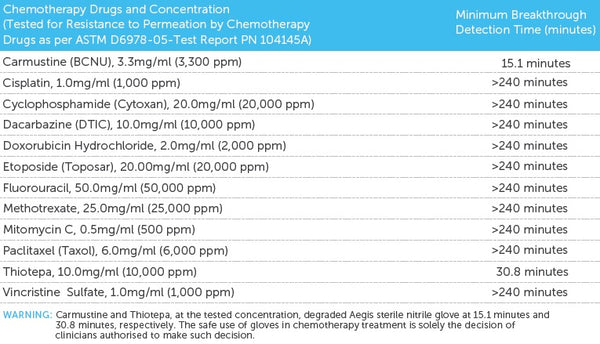
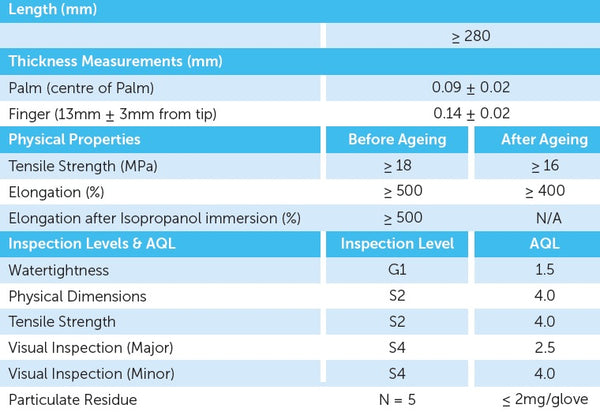
Aegis, like an armour, offers superb hand barrier protection and it dons like a second skin. The extended cuff length provides extra protection, high tensile strength and resistance to chemicals as expected from a high performance nitrile glove. This is our recommended sterile exam glove for procedures and jobs that demand tactile sensitivity.
Features:
- Pair
- Individual peel sterile pair peel packs
- Extra-long cuff extension 28cm
- Maximum protection
- Powder free
- Textured for enhanced grip
- Undetectable residual chemicals
- Biodegradable
- Zero protein
- Made to the number 1 grade of international glove level (grades 1-6)
- Perfect fit for exceptional sensitivity
- Unique flat pack ensures gloves dispense one at a time.
- Best value because staff have less tears so no wastage.
- Reduced skin reactions
- High strength & tear resistance
- Anatomically designed to reduce hand fatigue
- Complies with AZ/NZS 4011-1997 & ASTM5712-99 Standards
- Registered TGA with ARTG Number 203002
Regulatory Compliance
TGA - ARTG 203002, FDA 510(k), MDD 93/42/EEC, REACH, ROHS Directive 2002/95/EC, EU 10/2011, EC 1935/2004, PPE 89/686/EEC
Standards
AS/NZS 4011.1:2014, ASTM D6319, ASTM D6978, CEN/TS 14234, EN 374 part
1, 2 & 3, EN 420, EN 455 part 1,2,3 & 4, EN 1186, EN 13130
Manufacturing Accreditations
ISO 9001:2015, ISO 13485:2003, EN ISO 13485:2012
SOURCE - SUPPLY - PROTECT
Returns Policy
There is a 30 day return on any faulty products. To be eligible you must return items at your cost. We guarantee that any product found to be faulty supplied by us, will be accepted for return and we will refund all costs associated with the returned item. Statutory Warranties and conditions of Sale apply in Australia under consumer law for all products supplied by Australian Companies.
IMPORTANT NOTE: Should any faulty item be a TGA Registered Medical Product or Device, we are required to submit a report to the Registered Manufacturer in accordance with the TGA Legislation. This is a way of ensuring that any device is safely tracked and monitored for safety. We may ask you a few questions and we are required to verify any fault. All standard State & Federal Warranty Conditions apply.
Shipping
SOLMED only supplies to Australian Addresses.
Solmed dispatches all goods via Australia Post or TNT whichever is deemed suitable. We post to all addresses in Australia including Post Office Boxes. Tracking will be notified on Parcels where applicable. Solmed will advise if any product is not available and offer either a substitute, refund or back order.