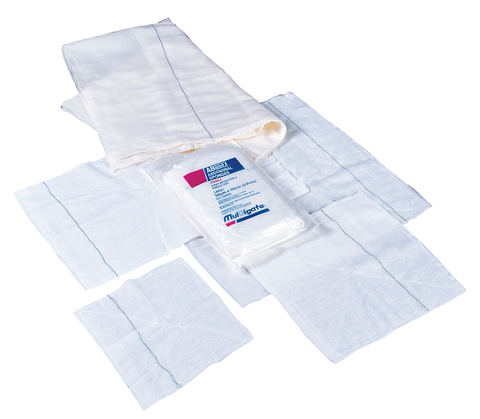
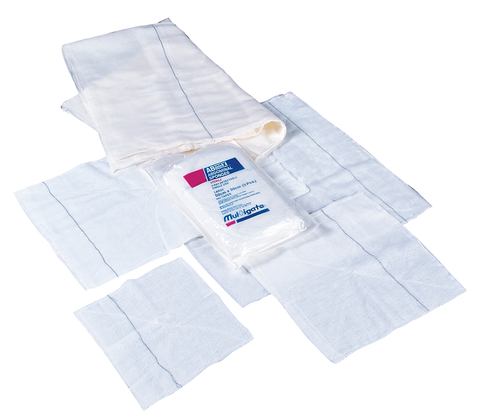
MULTIGATE COMBINE DRESSING FIRST AID ROLL WOUND CARE 10CM X 10 Metres
SOURCE - SUPPLY - PROTECT
4 TIMES MORE ABSOBENT THAN GAUZE & WOOD PULP
Ideal for GP’s, Beauty, Home, Artists, First Aid Kits etc
TGA 218578
Well known Multigate Brand,
Used throughout Hospitals Worldwide
Non Woven super quality
These Premium Non Woven Combines from Multigate consist of a wadding of mixed viscose/polyester fibres enclosed
by a non woven polyester cover. The mixed fibres are confined within the cover by heat sealing.
The ends of the cover and the two edges along the length of the combine are glued.
The absorbent wadding of the non woven combines are made from mixed fibres of 80% Polyester/ 20% Viscose.
The cover enclosing the wadding is nonwoven polyester fabric.
The weight of the non woven fabric is 18gsm.
A synthetic resin is used to bind the overlap in the non-woven covers along the length on one side of combine.
The carded polyester/viscose fibres are then cut to form wadding of the required size.
A non-woven polyester fabric covers the absorbent wadding.
The ends of the fabric are heat sealed to contain the wadding.
The fabric overlaps the wadding along its length and then sealed with synthetic resin green colour to prevent exposed fibres from the wadding.
Physical characteristics of the non woven swabs are white to off white in colour, minimal lint and with no visible contamination.
Returns Policy
1. Prior Approval Required: All returns must be approved by us prior to sending any goods back. Please contact us to obtain a return authorisation before returning any items.
2. Claims for Issues: Claims for short delivery, incorrect supply, or damaged goods during transport must be made within 7 days of the delivery date. Please inspect your goods upon receipt and notify us immediately if there are any issues.
3. Return Conditions: We generally do not accept returns for any reason other than a faulty product. This includes returns due to change of mind or incorrect orders However, at our discretion and depending on the product, we may accept non-faulty returns. These will incur a 10% restocking fee based on the product's purchase price. Refunds will be processed for the remainder of the order minus the original shipping cost and restocking fee, after the item has been inspected and approved. The buyer is responsible for returning the item in the same condition it was received. We do not accept returns for items that are damaged, opened, or not in original condition.
4. Lost Returns: If a returned item is lost in transit from the buyer to Solmed, we will not be held responsible. Please ensure that you use a trackable and insured shipping method..
5. TGA Duties: For products regulated by the Therapeutic Goods Administration (TGA), returns must comply with relevant TGA regulations and guidelines. Any return of TGA-regulated products will be subject to additional conditions as required by law.
6. Return Shipping Costs: Return shipping costs are the responsibility of the buyer unless otherwise agreed upon in the case of faulty products.
7. If you have any questions regarding returns or need assistance, please contact us before proceeding with the return process.
Shipping
1. Responsibility for Delivery: Once your parcel is with the courier service, it is beyond our control. We are not responsible for any loss, damage, or delivery issues that may occur once the parcel is in the possession of the courier.
2. Authority to Leave: All orders are dispatched without the requirement of a signature. The courier may leave your parcel in a location they deem to be a “safe place.” We cannot be held liable for any loss, damage, or theft that may arise from this decision.
3.Signature on Delivery: For added security, you may select the Signature on Delivery option at checkout. This ensures your parcel will not be left unattended. If no one is available to receive it, the parcel will be taken to the nearest post office or courier depot for collection.
4. Tracking Information: Tracking numbers will be provided to you via email. It is the buyer’s responsibility to monitor the progress of their parcel using the tracking information provided.
5. Buyer’s Responsibility: Any discrepancies or issues with the delivery must be followed up with the respective courier service by the buyer.
6. Transit Cover (Optional): You may purchase transit cover to insure the value of your order in case of loss, damage, or theft. The cost is 1% of your order total. Please contact us if you’d like to add this to your order. This is the only option that provides coverage for your order value.