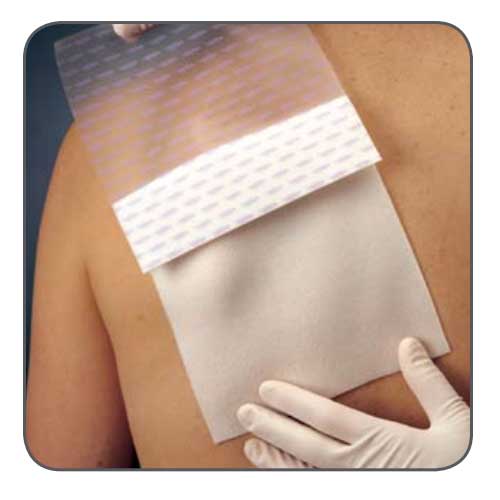
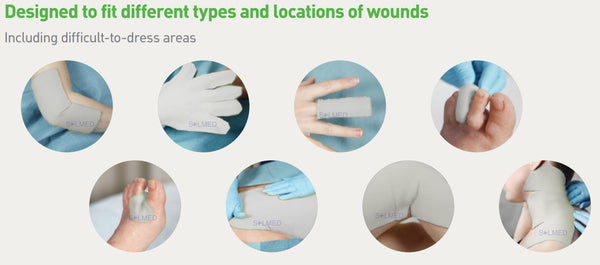
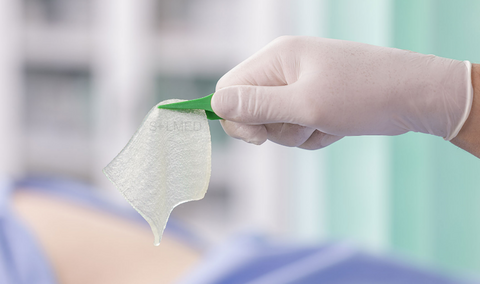
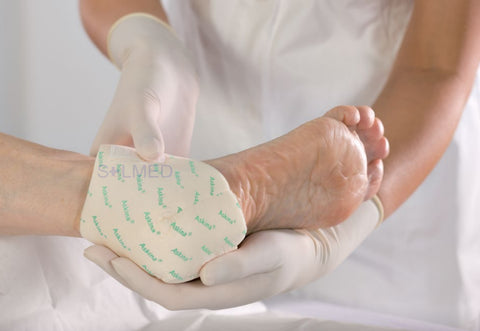
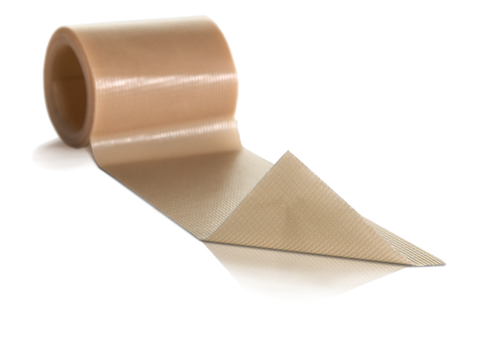
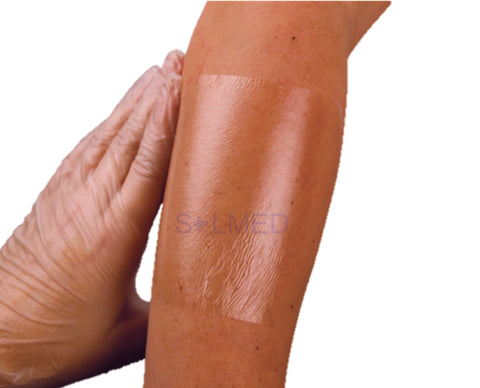
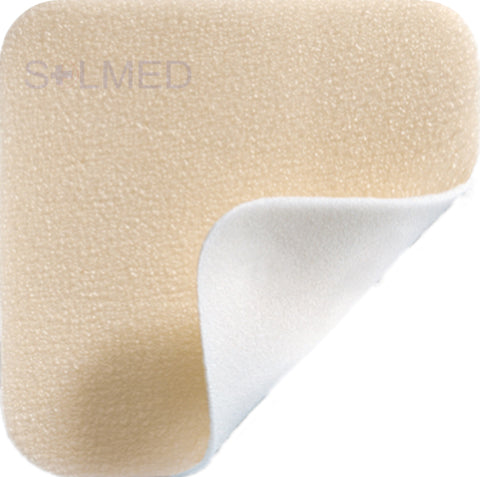
Mepilex Transfer foam exudate transfer layer is designed to be used as a primary wound contact dressing. The structure draws exudate from the wound, transferring the fluid to a secondary absorbent dressing. This combination of dressings maintains a moist wound environment. Mepilex Transfer dressing is soft, thin and highly conformable. So, it's easy to keep the dressing in contact with the wound surface and the surrounding skin, even in awkward or difficult-to-dress areas. The dressing moulds softly to skin without sticking to the moist wound through Safetac technology.
Less Pain & Trauma
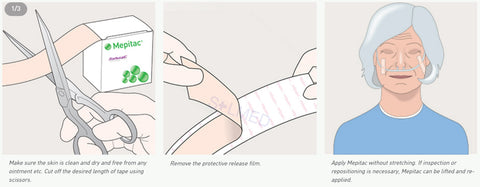
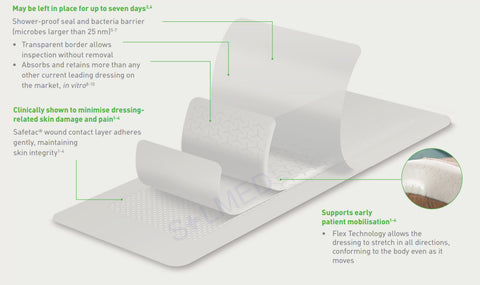
Safetac is a proprietary adhesive technology that minimises pain to patients and trauma to wounds. Designed to mold softly to the moist wound without sticking, so it can be removed easily without damaging the wound bed or skin to support faster natural healing. It also seals the wound margins to protect skin from damaging leaks and maceration. Safetac technology layer seals around the wound and the foam structure of Mepilex Transfer Ag allows exudate to move vertically into a secondary absorbent dressing.
Benefits
- Transfers exudate away from the wound
- Atraumatic to the wound and surrounding skin on removal
- Conforms well to body contours
- May be cut to shape
- Minimises the risk of maceration
- Promotes patient comfort during wear
- Minimises pain upon removal
Areas of Use
- Moderate to high exuding wounds
- Difficult-to-dress wounds
- As a protective layer on non-exuding wounds
- Large areas with fragile skin
- Can be used under compression
Resources
Returns Policy
1. Prior Approval Required: All returns must be approved by us prior to sending any goods back. Please contact us to obtain a return authorisation before returning any items.
2. Claims for Issues: Claims for short delivery, incorrect supply, or damaged goods during transport must be made within 7 days of the delivery date. Please inspect your goods upon receipt and notify us immediately if there are any issues.
3. Return Conditions: We generally do not accept returns for any reason other than a faulty product. This includes returns due to change of mind or incorrect orders However, at our discretion and depending on the product, we may accept non-faulty returns. These will incur a 10% restocking fee based on the product's purchase price. Refunds will be processed for the remainder of the order minus the original shipping cost and restocking fee, after the item has been inspected and approved. The buyer is responsible for returning the item in the same condition it was received. We do not accept returns for items that are damaged, opened, or not in original condition.
4. Lost Returns: If a returned item is lost in transit from the buyer to Solmed, we will not be held responsible. Please ensure that you use a trackable and insured shipping method..
5. TGA Duties: For products regulated by the Therapeutic Goods Administration (TGA), returns must comply with relevant TGA regulations and guidelines. Any return of TGA-regulated products will be subject to additional conditions as required by law.
6. Return Shipping Costs: Return shipping costs are the responsibility of the buyer unless otherwise agreed upon in the case of faulty products.
7. If you have any questions regarding returns or need assistance, please contact us before proceeding with the return process.
Shipping
1. Responsibility for Delivery: Once your parcel is with the courier service, it is beyond our control. We are not responsible for any loss, damage, or delivery issues that may occur once the parcel is in the possession of the courier.
2. Authority to Leave: All orders are dispatched without the requirement of a signature. The courier may leave your parcel in a location they deem to be a “safe place.” We cannot be held liable for any loss, damage, or theft that may arise from this decision.
3.Signature on Delivery: For added security, you may select the Signature on Delivery option at checkout. This ensures your parcel will not be left unattended. If no one is available to receive it, the parcel will be taken to the nearest post office or courier depot for collection.
4. Tracking Information: Tracking numbers will be provided to you via email. It is the buyer’s responsibility to monitor the progress of their parcel using the tracking information provided.
5. Buyer’s Responsibility: Any discrepancies or issues with the delivery must be followed up with the respective courier service by the buyer.
6. Transit Cover (Optional): You may purchase transit cover to insure the value of your order in case of loss, damage, or theft. The cost is 1% of your order total. Please contact us if you’d like to add this to your order. This is the only option that provides coverage for your order value.