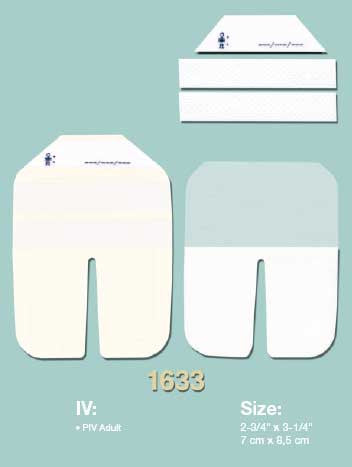
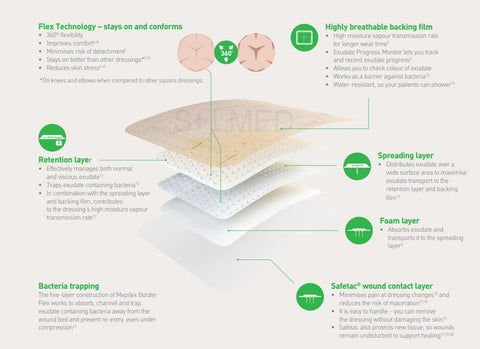
Osmocel Si Silicone Hydroporous Foam dressings are multi-layered wound dressings, comprising of a silicone wound and skin contact layer, white polyurethane foam (and superabsorbent polymer layer*) exudate management layer, backed with a skin-toned, polyurethane film support layer.
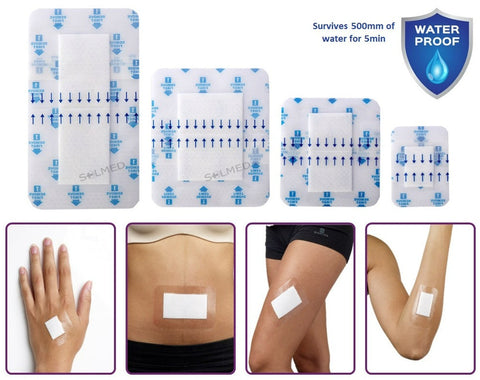
Osmocel Si Silicone Hydroporous Foam readily absorbs exudate, producing an optimal moist wound healing environment with the added benefit of atraumatic removal. Osmocel Si Silicone Hydroporous Foam has in-built Moisture Vapour Transmission properties, combined with fluid absorption capacities, that make it suitable for the management of wounds with light to moderate levels of exudate.
In the presence of moisture, the non-adherent property of the silicone layer of Osmocel Si Silicone Hydroporous Foam prevents adherence to the wound, newly formed tissue and surrounding fragile, or sensitive skin. This ensures dressing changes can take place with minimal tissue disturbance.
The outer backing of Osmocel Si Silicone Hydroporous Foam Bordered dressing is water waterproof.
Each dressing is individually packed, ready for use, and sterilised by ethylene oxide. Osmocel Si Silicone Hydroporous Foam dressings are sterile unless the package is opened or damaged, are single use only and should not be re-sterilised.
General Information:
- Prescribed compression treatment for Venous Leg Ulcer Management can be continued while using Osmocel Si Silicone Hydroporous Foam.
- If infection is present, a primary wound cleansing dressing e.g. Osmonate® Calcium Alginate dressing or rope, or an antimicrobial layer, such as Zorflex® Wound Contact Layer should be used in combination with Osmocel Si Silicone Hydroporous Foam. The clinician in-charge is responsible for determining if necessary.
- No known side effects have been observed or reported in the use of Osmocel Si Silicone Hydroporous Foam dressings.
- Observe for wound infection. Consult the clinician-incharge if any of the following occurs: fever, increased pain, redness, swelling, abnormal smell or exudate.
Indications:
Osmocel Si Silicone Hydroporous Foam dressings are indicated for use as primary dressings in the management of all types of superficial wounds with light to moderate exudate production, such as:
- Skin tears
- Partial thickness burns
- Pressure injuries
- Arterial ulcers
- Venous leg ulcers
- Diabetic ulcers
Surgical wounds or as a secondary dressing with other wound contact products, where exposure to conventional adhesive fixation is undesirable.
Contraindications:
- Should not be used if allergies to any of its components is known.
- Not suitable for full thickness burns and wounds before bleeding has ceased.
- Do not simultaneously use Osmocel Si Silicone Hydroporous Foam with an oxidant, e.g hydrogen peroxide or chlorate solution. This may affect the structure and performance of the dressing
Dressing Change:
The clinician-in-charge is responsible for determining the need for dressing changes, dependent on the stage and phase of wound healing and exudate level. The dressing should be changed when exudate absorption is considered to be extending beyond the wound surface area. 7 days is the maximum period between dressing changes.
Returns Policy
1. Prior Approval Required: All returns must be approved by us prior to sending any goods back. Please contact us to obtain a return authorization before returning any items.
2. Claims for Issues: Claims for short delivery, incorrect supply, or damaged goods during transport must be made within 7 days of the delivery date. Please inspect your goods upon receipt and notify us immediately if there are any issues.
3. Return Conditions: We do not accept returns for any reason other than a faulty product. This includes, but is not limited to, returns due to change of mind or incorrect orders.
4. TGA Duties: For products regulated by the Therapeutic Goods Administration (TGA), returns must comply with relevant TGA regulations and guidelines. Any return of TGA-regulated products will be subject to additional conditions as required by law.
5. Return Shipping Costs: Return shipping costs are the responsibility of the buyer unless otherwise agreed upon in the case of faulty products.
6. If you have any questions regarding returns or need assistance, please contact us before proceeding with the return process.
Shipping
1. Responsibility for Delivery: Once your parcel is with the courier service, it is beyond our control. We are not responsible for any loss, damage, or delivery issues that may occur once the parcel is in the possession of the courier.
2. Safe Place Deliveries: If the courier decides to leave your parcel in a location they deem as a “safe place,” we cannot be held liable for any issues that arise from this decision.
3.Signature Option: For added security, you may request a signature upon delivery for an additional fee of $3.00 per carton. This option ensures that your parcel will not be left unattended and, if no one is available to receive it, will be taken to the nearest post office or depot for pickup.
4. Tracking Information: Tracking numbers will be provided to you via email. It is the buyer’s responsibility to monitor the progress of their parcel using the tracking information provided.
5. Buyer’s Responsibility: Any discrepancies or issues with the delivery must be followed up with the respective courier service by the buyer.